
A couple years ago I did a project where I looked for biological models that might help answer the question, How does nature adapt to increasing scarcity of water, nutrients and energy? In light of the Biomimicry Institute’s 2017 Global Design Challenge focus on climate change, I thought I’d share what I found in the hopes it inspires the innovation we so urgently need in this area.
Aside from two weeks of frigid temperatures and snow in December, I’m not sure winter is coming to Chicago this year. People I talk to around the world have anecdotes that are similar – weather patterns are just not the same. And as we all know, despite our current government’s attempt to silence its own scientists, the notion of climate change does not rest on anecdotes but on solid science. And the science tells us that climate change is accelerating. Whether we choose to “believe” it or not is irrelevant. The question is, what are we going to do about it? (For a description of how humans are impacting global climate cycles, see my previous post.)
To be a part of the solution, the Biomimicry Institute’s 2017 Global Design Challenge (currently ongoing – deadline April 30!) is focused on climate change. They have two general categories for which they are looking for innovations:
- Helping communities adapt to or mitigate climate change impacts (i.e. those forecasted or already in motion), and/or
- Reversing or slowing climate change itself (e.g. by removing excess greenhouse gasses from the atmosphere).
These are not small challenges. Where does one start?
Because these problems are so complex and will require systemic changes that are comprised of behavioral changes from the individual to system levels, breaking down the challenge into more specific areas of inquiry can help challenge teams focus on biologized questions that might give a team natural models that will inspire concrete results. When brainstorming natural models it’s important to look at all scales, from single organisms to ecosystems. Diversity in scale and context can begin to provide a better picture of how individual behaviors add up to system dynamics, and potentially give innovative insights into critical leverage points in the system.
A sampling of biological models for adaptation
A couple years ago I did a team project where we looked for biological models that might help answer the question, How does nature adapt to increasing scarcity of water, nutrients and energy? I came up with the following natural models and design principles. I hope these might help you and your team in your challenge innovation process!
Click on the title of each box to link to summaries of the strategies and key references I used to develop these design principles provided farther below. Please feel free to reach out to me for more information on any of these biological models.
Photosynthetic Plasticity Enables Rapid Acclimation: CAM Plants
Biological strategy
Photosynthetic plasticity enables CAM plants to acclimatize in response to dynamic environments through a variety of rapid, flexible and reversible photosynthetic processes.
Design Principle
Employ a variety of rapid, flexible and reversible resource management methods that activate or deactivate in response to short-term variations in resource availability enabling survival during periods of scarcity and exploitation of resources during periods of abundance.
Phenotypic Plasticity Maintains Fitness: Dandelion
Biological strategy
Phenotypic plasticity of the dandelion maintains fitness across a wide variety of light intensity environments by modifying structural, chemical and reproductive traits in response to local light levels.
Design Principle
Modifying structural, chemical and growth mechanisms in response to energy intensity levels enables optimized access to and use of available energy resources across a wide variety of systems.
Plant Maximizes Water Utilization: Creosote
Biological strategy
The Creosote bush maximizes water utilization by adjusting growth patterns, chemical processes and allocation of resources to optimally maintain function in response to unpredictable water availability, enabling survival under severe drought.
Design Principle
Maximize resource utilization by adjusting growth patterns, vital functional processes and allocation of resources to optimally maintain function in response to unpredictable resource availability, enabling survival under severe resource scarcity.
Native Symbiotic Structures Increase Fitness: Fungi
Biological strategy
Plants and fungi co-adapted in nutrient-poor native soils increase their fitness by developing more expansive symbiotic structures to maximize uptake and exchange of scarce resource(s) most limiting to growth.
Design Principle
Local entities adapted to resource-limited environments increase success by developing more expansive symbiotic local resource uptake networks and exchange nodules specifically designed to maximize access to and availability of the local resource(s) most limiting to growth.
System Development Process Secures Scarce Nutrients: Ecological Succession
Biological strategy
The process of ecological succession secures increasingly scarce nutrients by establishing progressively complex interdependent structures and processes that increasingly minimize nutrient outflow and lock up nutrients in organic matter that stays within the system, resulting in a closed-loop nutrient cycling system.
Design Principle
Secure increasingly scarce resources by establishing progressively complex interdependent system components that increasingly minimize resource outflow and incorporate resources into system component materials that do not leave the system, resulting in a closed-loop resource cycling system.
CAM Plants
 While most plants open their pores during the day to absorb carbon dioxide (CO2) from the air to conduct photosynthesis (converting sunlight energy to sugars needed for growth) in a process called C3, CAM plants open up their pores and take in CO2 at night when conditions are cooler and more humid to reduce by approximately one-tenth the amount of water loss that occurs when pores are open. CAM plants then store the CO2 until daylight when photosynthesis occurs in sunlight behind closed pores. There are four phases identified in the CAM process, and depending on environmental conditions, CAM plants may employ all four phases including opening their pores in early and late daytime light (in times of sufficient water) or only one phase when the pores never open day or night (in severely dry conditions). CAM plants from desert habitats, such as cactuses and succulents, where intense permanent stressors are intense heat, sunlight and lack of water, have evolved very little variation in their use of CAM – even when moved to more humid habitats they still open their pores only at night.
While most plants open their pores during the day to absorb carbon dioxide (CO2) from the air to conduct photosynthesis (converting sunlight energy to sugars needed for growth) in a process called C3, CAM plants open up their pores and take in CO2 at night when conditions are cooler and more humid to reduce by approximately one-tenth the amount of water loss that occurs when pores are open. CAM plants then store the CO2 until daylight when photosynthesis occurs in sunlight behind closed pores. There are four phases identified in the CAM process, and depending on environmental conditions, CAM plants may employ all four phases including opening their pores in early and late daytime light (in times of sufficient water) or only one phase when the pores never open day or night (in severely dry conditions). CAM plants from desert habitats, such as cactuses and succulents, where intense permanent stressors are intense heat, sunlight and lack of water, have evolved very little variation in their use of CAM – even when moved to more humid habitats they still open their pores only at night.
However, CAM plants adapted to dynamic environments where conditions are variable, such as orchids living on tree branches in the tree canopy in tropical forests, have the ability to modify the CAM phase they are expressing in response to changing environmental stresses, sometimes within a matter of hours. Some CAM plants (CAM/C3 intermediates) are also able to switch completely from using the CAM process to using the C3 photosynthetic process in response to environmental conditions. Stressors in the environment which might trigger changes in process include interactions between temperature, sunlight, water, carbon dioxide, nutrients and sometimes salinity. The photosynthetic plasticity of tropical CAM plants allows fast, flexible and readily reversible responses to allow for acclimation in response to dynamic stresses enabling CAM plants to occupy many niches with high species diversity in a wide range of tropical habitats where resource availability is variable.
References
Lu¨ ttge U. 2010. Ability of crassulacean acid metabolism plants to overcome interacting stresses in tropical environments. AoB PLANTS 2010: plq005, doi:10.1093/aobpla/plq005. Epub 2010 May 13. doi: 10.1093/aobpla/plq005
Borland, et.a al. 2011. The photosynthetic plasticity of crassulacean acid metabolism: an evolutionary innovation for sustainable productivity in a changing world. New Phytol. 2011 Aug;191(3):619-33. doi:10.1111/j.1469-8137.2011.03781.x. Epub 2011 Jun 16.
Kluge, M., Razanoelisoa, B. and Brulfert, J. (2001), Implications of Genotypic Diversity and Phenotypic Plasticity in the Ecophysiological Success of CAM Plants, Examined by Studies on the Vegetation of Madagascar1. Plant Biology, 3: 214–222. doi:10.1055/s-2001-15197
Osmond, CB. 1978. Crassulacean Acid Metabolism: A Curiosity in Context. Annual Review of Plant Physiology. Vol.29:379-414 (Volume publication date June 1978) doi:10.1146/annurev.pp.29.060178.002115
Dandelion
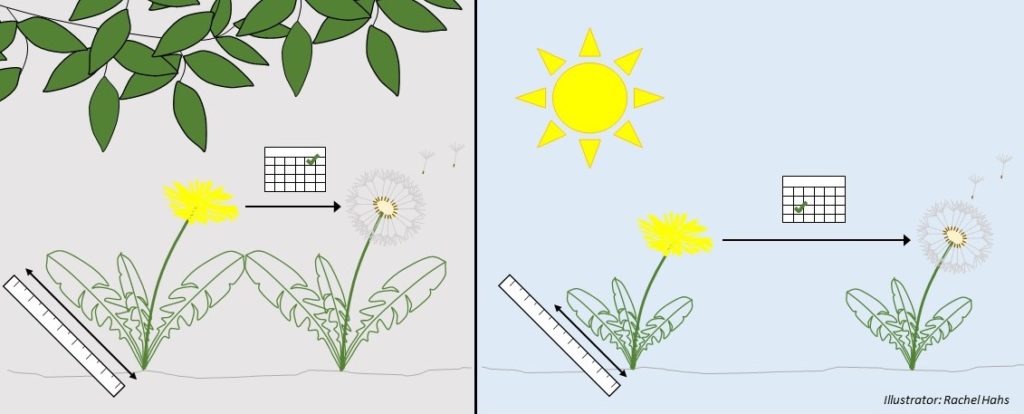
Dandelions are able to survive under a wide variety of environments. Dandelions most often produce seeds without needing a pollinator, resulting in offspring that are clones (genetically identical) of the parent plant. Therefore, their ability to be significantly adaptive to local environments rests not in changes occurring at the genetic level but as a result of their phenotypic plasticity – i.e., their ability to change their structural and functional traits in response to the limiting (scarce) resources in a local environment. Trait changes may include altering traits such as photosynthetic processes, leaf angles, and flowering time. In response to decreasing availability of light in shaded understories, dandelions produce significantly longer, more rounded “shade” leaves with a greater surface area to collect scarce light resources; taller flower stems to increase access to potential pollinators; and they speed up the time it takes to flower, create and disperse mature seeds.
References
Molina-Montenegro, M.A., Peñuelas, J., Munné-Bosch, S. et al. “Higher plasticity in ecophysiological traits enhances the performance and invasion success of Taraxacum officinale (dandelion) in alpine environments.” Biol Invasions (2012) 14: 21. doi:10.1007/s10530-011-0055-2
Creosote
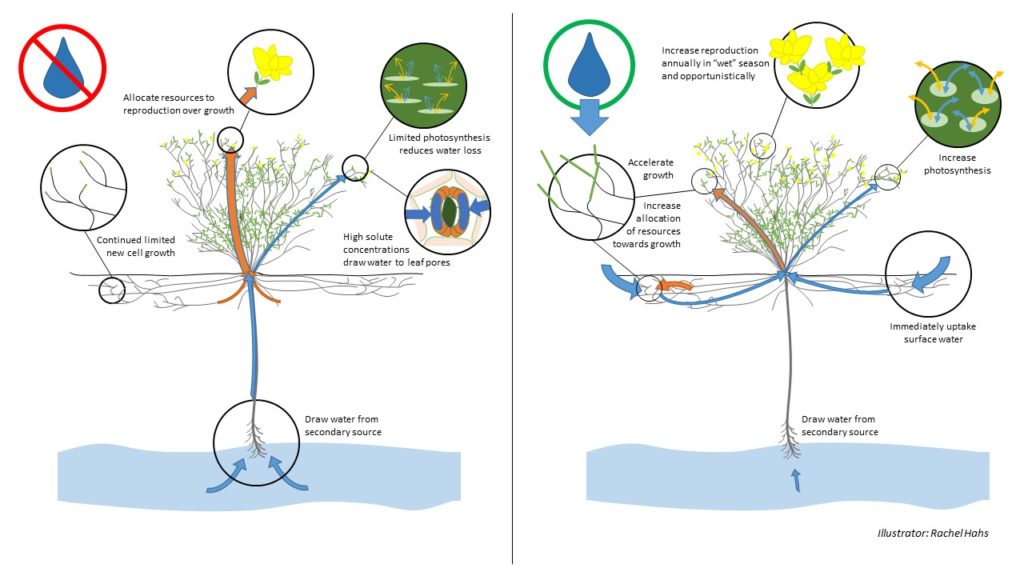 Creosote is the most drought-tolerant plant in North America, using a wide range of adaptations to live in harsh habitats where sometimes it is the only plant living. Creosote can live with no rain at all for more than two years and individuals can live to be thousands of years old. Creosote essentially maintains two mechanisms for growth and reproduction to maximize utilization of a scarce resource – through an annual growing season when rains are typically present (in the spring), and opportunistically when brief rains occur at other times of the year.
Creosote is the most drought-tolerant plant in North America, using a wide range of adaptations to live in harsh habitats where sometimes it is the only plant living. Creosote can live with no rain at all for more than two years and individuals can live to be thousands of years old. Creosote essentially maintains two mechanisms for growth and reproduction to maximize utilization of a scarce resource – through an annual growing season when rains are typically present (in the spring), and opportunistically when brief rains occur at other times of the year.
Under extremely dry conditions, Creosote is able to continue to grow and reproduce with minimal water loss. In decreasing water availability, the bush is able to adjust its osmotic potential significantly lower, meaning it increases chemical concentrations in its leaves which increases its ability to move water from soil to roots to leaves (but is also taxing on the plant). The extremely low osmotic potential enables the bush to draw water up to its leaves from underground sources through its deep “tap” root (up to about 10 feet in depth). With a low osmotic potential the leaf cells are able to maintain enough water to continue with reduced photosynthesis such that the plant continues to grow and even flower in drought, albeit in a limited capacity. In periods of water stress Creosote allocates resources to reproduction at a cost to vegetative growth.
However, the Creosote bush is also able to quickly capitalize on brief rains when they occur. Creosote has a second thin fibrous root pattern that exists just under the soil surface that can spread out up to 50 square yards from the base of the plant. New cell growth on these surface roots are incredibly efficient at soaking up surface water, appearing to grow inches in day. With the sudden increase in water availability, Creosote is able to adjust its osmotic potential appropriately and increase photosynthesis and thus growth, including a quick burst of flowering. In addition, the bush will continue at an accelerated growth rate while water is available, allocating additional resources towards vegetative growth. Because Creosote blooms both seasonally and opportunistically, plants during late summer monsoons can be putting out new shoots, blooming, and setting seed at the same time. Once the water is gone, the plant will again adjust growth and chemical processes to withstand drought conditions.
References
MEINZER, F. C., RUNDEL, P. W., SHARIFI, M. R. and NILSEN, E. T. (1986), Turgor and osmotic relations of the desert shrub Larrea tridentata. Plant, Cell & Environment, 9: 467–475. doi:10.1111/j.1365-3040.1986.tb01762.x
Sharifi, M. R., et al. “Effect of Manipulation of Water and Nitrogen Supplies on the Quantitative Phenology of Larrea Tridentata (Creosote Bush) in the Sonoran Desert of California.” American Journal of Botany, vol. 75, no. 8, 1988, pp. 1163–1174., www.jstor.org/stable/2444099.
Mycorrhizal Fungi
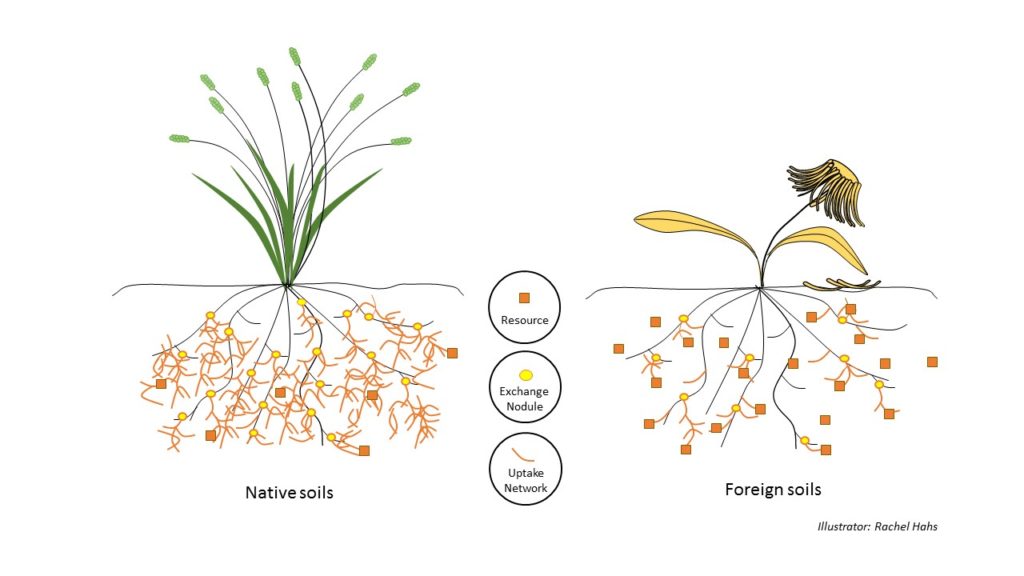 Plants and fungi that co-adapt in nutrient-poor native soils improve their survival and reproduction by developing larger mutually beneficial structures to maximize uptake and exchange of the locally scarce resource(s) that are most limiting to growth. Fungi grown in the soils in which they have evolved grow larger fibrous branching growth structures for nutrient uptake and many more resource exchange nodules on the roots of plants also adapted to the same local nutrient-poor soils. These fungi structures are specifically adapted to maximize the exchange of the limiting resources in their local soil conditions. For example, if phosphorus is scarce in home soils, the fungi develop large structures to maximize the uptake and exchange of phosphorus with their host plants. However, if this same fungi were moved to a foreign soil location where nitrogen was scarce and most limiting to growth, the fungi would not only develop fewer growth and exchange structures, but would still maximize uptake of phosphorus, thereby not maximizing the survival and reproduction of plants deficient in nitrogen in the foreign soil. Therefore, survival and reproduction of plants and fungi is greatly increased when plants and fungi are grown together in their home soils because of their strategy to mutually maximize the uptake and exchange the locally limiting resource(s).
Plants and fungi that co-adapt in nutrient-poor native soils improve their survival and reproduction by developing larger mutually beneficial structures to maximize uptake and exchange of the locally scarce resource(s) that are most limiting to growth. Fungi grown in the soils in which they have evolved grow larger fibrous branching growth structures for nutrient uptake and many more resource exchange nodules on the roots of plants also adapted to the same local nutrient-poor soils. These fungi structures are specifically adapted to maximize the exchange of the limiting resources in their local soil conditions. For example, if phosphorus is scarce in home soils, the fungi develop large structures to maximize the uptake and exchange of phosphorus with their host plants. However, if this same fungi were moved to a foreign soil location where nitrogen was scarce and most limiting to growth, the fungi would not only develop fewer growth and exchange structures, but would still maximize uptake of phosphorus, thereby not maximizing the survival and reproduction of plants deficient in nitrogen in the foreign soil. Therefore, survival and reproduction of plants and fungi is greatly increased when plants and fungi are grown together in their home soils because of their strategy to mutually maximize the uptake and exchange the locally limiting resource(s).
References
Collins Johnson, Nancy, et al. “Resource limitation is a driver of local adaptation in mycorrhizal symbioses.” PNAS, vol 107 no. 5, February 2, 2010, pp.2093-2098. doi: 10.1073/pnas.0906710107
Klironomos, J. N. (2003), Variation in Plant Response to Native and Exotic Arbuscular Mycorrhizal Fungi. Ecology, 84: 2292–2301. doi:10.1890/02-0413
Ecological Succession

Ecosystem development following a disturbance where no life is present (such as after lava flows) is called primary succession. At the start of primary succession, nutrients necessary for plant life are locked up in the bedrock (such as phosphorus) or in the air (such as nitrogen). Pioneering plants that are first on the scene are able to tap into these nutrients by fracturing and wedging into rocks with their root systems. As soils develop around the plant roots from a buildup of decaying plant and weathered rock materials, these pioneering plants also fix nitrogen from the air to the soils. At this stage, however, a high rate of decomposition due to higher temperatures from sun exposure breaks down nutrients in the system at a high rate. Many of the nutrients gathered on site are lost in surface water runoff and leaching as the soils are shallow and not well compacted. As additional abundant quick-growing, quick to seed plants are established, the increasing soil layer serves to reduce water runoff and promote the slow percolation of water, increasing the availability of water and nutrients. Decomposition (breaking down) of organic matter, plant roots and soil organisms add carbon dioxide to soils, helping to increase the acidity of soils which serves to break down minerals into nutrients available for uptake by plants.
Once enough nitrogen is present in soils, an additional diversity of plants start to grow on site. Increasing diversity of plant (and animal) life results in an increasing variety and density of physical structures including leaf sizes, height of plants, and roots structures. The more complex system structure is increasingly able to absorb additional gases from the air while also creating deeper, more compact soils that trap nutrients on site. In addition, with the increasing canopy of leaves and larger root volumes, water runoff is again decreased not only because the soils are able to hold more water, but because the plants also return water as vapor to the air, decreasing the amount of water making it down to the soil and flowing off site. Decreased runoff decreases the loss of nutrients. However, due to the high rate of nutrient loss (particularly phosphorus) during primary succession, and as more species colonize the site, fewer nutrients are available relative to the increasing organic matter on site. The increasing resource scarcity results in increasing competition as well as mutualisms between organisms to gain access to increasingly scarce nutrients. Plants that grow more slowly and are able to capture nutrients in their roots and mass, such as shrubs and trees, gain competitive advantage and push out the smaller, faster-growing, nitrogen-fixing plant species.
As ecological succession continues toward a mature state where species diversity is greatest but nutrients limiting to growth are scarce, nutrients become locked up in the soils and plant and animal matter, with the highest amount of nutrients locked up in decomposing matter like leaf litter. At this stage the ecosystem structure has mature trees that block much of the sunlight making it down to the forest floor, which slows the rate of decomposition as well as reduces water loss. The rarest of nutrients necessary for plant growth, such as phosphorus, are cycled tightly within the ecosystem’s physical and chemical structures. This type of nutrient cycling is considered “closed loop”, meaning the loss of nutrients is minimized and the diverse array of species in all niches of the ecosystem take part in the upcycle of nutrients throughout the system again and again. By locking up scarce resources necessary for life within the ecosystem structure, the system avoids collapse.
References
Allenby, B. R. and Cooper, W. E. (1994), Understanding industrial ecology from a biological systems perspective. Environ. Qual. Manage., 3: 343–354. doi:10.1002/tqem.3310030310
Gorham, Eville, et al. “The Regulation of Chemical Budgets Over the Course of Terrestrial Ecosystem Succession.” Annual Review of Ecology and Systematics, vol. 10, 1979, pp. 53–84., www.jstor.org/stable/2096785.
You should check out http://www.pterofin.com
Fascinating ! As Janine says: nature has it all figured out!!!
The Biomimicry Global Design Challenge is an annual competition that asks teams of students and professionals to address critical global issues with nature-inspired solutions.